One of the most basic rules for playing the game “Twenty Questions” is that all of the questions must be definitively answered by either “yes” or “no.” The exchange of information allows the players to correctly guess the item in question.

Igor Grigoriev and other DOE JGI Fungal Program collaborators appear in a short video at http://bit.ly/JGI-Fungal-vid.
Fungal researchers have been using a variation of Twenty Questions to determine if wood-decaying fungi fall under one of two general classes. If a fungus can break down all the components – cellulose, hemicellulose and lignin – of plant cell walls it is considered a white rot fungus. If a fungus can only break down cellulose and hemicellulose but not lignin, they classify it as a brown rot fungus. Known white rot fungi produce certain lignin-degrading enzymes called class II peroxidases (PODs) and a variety of enzymes that go after crystalline cellulose.
In a study published online the week of June 23, 2014 in the Proceedings of the National Academy of Sciences, a team led by U.S. Department of Energy Joint Genome Institute (DOE JGI) fungal researchers suggests that categorizing wood-decaying fungi as either white rot or brown rot may not be as clear-cut as previously thought. The discovery complicates but also broadens the range of fungal decay strategies to be explored for commercializing the process of biofuels production.
This finding emerged after researchers analyzed 33 basidiomycete fungal genomes, 22 of which are wood decayers, four of which had been recently sequenced by the DOE JGI. Based on previously sequenced genomes, the team observed that two of the new fungi, Botryobasidium botryosum and Jaapia argillacea, had the cellulose-attacking enzymes characteristic of white rot fungi, but lacked PODs, making them similar to brown rot fungi. Applying a statistical process called Principal Components Analysis (PCA) to find similarities in fungi based on their plant biomass degrading genes, they found that the two new fungi grouped close to Phanerochaete chrysosporium, the first white rot species sequenced. This was a curious finding because the new fungi were phylogenetically distant from P. chrysosporium, and, moreover, didn’t have PODs.
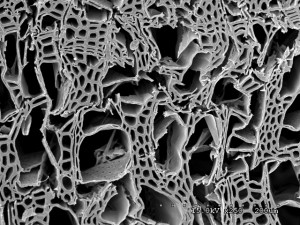
The white rot Botryobasidium botryosum was one of four fungi whose genomes were recently sequenced as part of the study published the week of June 23, 2014. The researchers grew the fungus on aspen wood and in localized areas, it broke down the cell walls and removed cellulose, hemicellulose and lignin, leaving large gaps in the structure. (Benjamin Held, University of Minnesota)
The team then grew isolates of B. botryosum and J. argillacea on pine and aspen wood. They found that the fungi superficially degraded the wood surfaces but in localized areas, went further and broke down the cell walls and removed cellulose, hemicellulose and lignin.
“[They] show similarities to white rot fungi in … all predicted carbohydrate- and lignin-active enzymes and can degrade all components of wood, but they do so without the PODs that are a hallmark of white rot,” the team reported in their paper. They also found a correlation between secondary metabolism genes, which are crucial for fungal survival, and brown rot fungi. These results, they added, suggest that the perceived dichotomy of white rot and brown rot is too simplistic and suggest that fungal wood decay capabilities be categorized instead on a continuum.
DOE JGI Fungal Genomics head Igor Grigoriev noted this wasn’t the first time they’d seen a genome that appeared to blur the definitions between white rots and brown rots. “We thought we saw an anomaly with a previously sequenced white rot fungus Schizophyllum commune,” he said. “Now we see a trend. This is the value of having multiple data points and so many fungal genome sequences. This is the whole point of doing fungal genomics at scale.”
Dan Eastwood, a fungal researcher at Swansea University who was not involved in the study, pointed out that fungi don’t have to follow rules to exhibit a decay form. “The manuscript is very timely and provides evidence for what many people in the field have suspected for some time – that simple descriptors of wood decomposition do not necessarily reflect the diversity in decay strategies exhibited by fungi,” he said. “This is particularly the case when discussing the brown rot wood decay mechanism where distantly related species have evolved superficially similar decay mechanisms. This manuscript uses whole genome sequence information to outline the argument for advancing our understanding of wood decomposition away from a simplistic white versus brown rot dichotomy.”
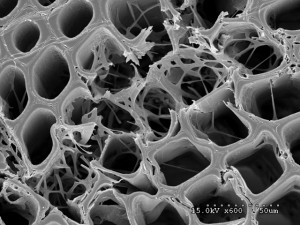
The white rot Jaapia argillacea was another of the four fungi whose genomes were recently sequenced as part of the study. It has characteristics of a white rot fungus but not all of the expected traits. Researchers grew the fungus on pine wood and it broke down the cell walls in localized areas, removing the cellulose, hemicellulose and lignin. (Benjamin Held, University of Minnesota)
“This is the first time we see patterns of white rot without this particular enzyme,” Grigoriev added. “That tells us these fungi degrade lignin using other means, which tells us POD is not the only marker for white rot. Now that it’s clear that it’s not the only player, we should broaden our search for enzymes that have bioenergy applications. It is important to identify a whole range of enzymes sourced from nature that can be used to develop second-generation biofuels in terms of breaking down lignin and other components in plant cell walls.”
Eastwood added that the work allows researchers to start to understand decay strategies in complex habitats. “Wood is a complex substrate in a complex environment,” he said. “Evolution of decay mechanisms will be complex also, and the diversity in gene compliment will reflect the polyphyletic nature of superficially similar decay strategies. The future challenges are to better define the chemical environment during wood decomposition in conjunction with enzyme activity of different species that appropriately reflect their specific ecology.”
For more information about the DOE JGI Fungal Program, view the short video at http://bit.ly/JGI-Fungal-vid.