This the second episode of Natural Prodcast, which continues a conversation from the first episode. You can go to part 3 here.
You can find the podcast on Apple, Google, Spotify, Stitcher, directly with the RSS link, and via most other commonly-used podcast feeds. Please subscribe, and leave a review!
If you have questions or comments, you can contact us on Twitter at @JGI or @danudwary, or by email to [email protected]. We’d love to get your feedback!
DAN: You’re listening to the US Department of Energy Joint Genome Institute’s “Natural Prodcast,” a podcast about the science and scientists of secondary metabolism.
DAN: Hi, again. This is Dan, and you’re listening to the second episode of the Natural Prodcast. This is part 2 of our “primer” episode, so if you ended up here somehow without having listened to the first part, you might be a little lost, and I’d suggest you go back and check the first episode out. This one continues the introductory conversation between myself and Alison, and we continue with the basic ideas of the science of natural products, and we dive into the very early history of the field. If you continue on, in the next episode you’ll hear us talk about the modern history of natural products and the state of the field today.
So, not as much of a preamble this time. Let’s jump right in.
—
DAN: So secondary metabolites. I kind of inferred through the stories I told you about geosmin, which is a bacterial sacred metabolite, and ergot alkaloids come from fungi, and Taxol from plants. Plants, fungi, bacteria, archaea, they all have secondary metabolism. They all have very developed secondary metabolism pathways. Not every organism, at least, has something that’s maybe obvious or notable. We are still in – not an early stage. I think we can identify most secondary metabolism pathways now, but there’s still almost certainly unusual things that we don’t recognize, because the only way to find things is by comparison. We’ll talk about that when we talk about molecular biology.
But um, yeah, so secondary metabolism is throughout nature, except maybe for animals and humans, not as much. You know, we have this human centric view of biology, normally, and so the things that we have, we wouldn’t consider to be secondary metabolites. You know, eye color is a pigment, right, but we don’t necessarily consider that a secondary metabolite because it’s something that we have. There are animals that are known to be sources of secondary metabolites, like sponges were a really common source for people to try to find new medicines for a long time. But we know now that most of those pathways come from bacteria that actually live within, say, the sponges or other things. And we know lots more about microbiomes and symbionts that live with other organisms, and often it’s the bacteria, or whatever, that lives with them that produces those. So it’s not a hard and fast rule, but, generally, animals do not have secondary metabolism that we really recognize, right?
ALISON: And I tend to think of microbes as being able to secrete a lot more things than people do…? Although I don’t study people so maybe I’m wrong. But yeah, I mean, because I think, is that a fair way to think about secondary metabolites as well? Well, I guess it’s not always, because it could be something that’s, it could be a molecule that’s embedded in their – what do you call that – I want to say like lipid bilayer, you know, in the cell.
DAN: It depends on lifestyle. So there are lots of different organisms that produce secondary metabolites for whatever reason. And so one of the ways I used to think about it when I was teaching is: bacteria might have different lifestyles. There are the kinds of bacteria that, maybe, grow in one place and sort of stay there. And then, you know, they will have to create an environment that’s good for them to grow in. They might have enough nutrients, but they might want to protect those nutrients with antibiotics. They might need to secrete siderophores to pick up metals and bring them back into their cell. And yeah, and then there are other bacteria that, maybe, are more motile and move around and don’t sort of “set up shop” in one place. And so those those guys that move around or swarm – they might be using secondary metabolites to produce chemicals that would give them the ability to talk to one another – to sort of know if their neighbors had already been to a location or something by leaving sort of a chemical trail the same way that, like, ants do. So, that’s also common as well. And yeah, so it really just depends on lifestyle.
ALISON: Okay. It’s such a dynamic part of their lives. Sounds like.
DAN: Literally, yeah. I mean, you might be producing things at different stages of growth or different signals from the environment might cause you to start producing things. Yeah.
ALISON: It sounds like it’s kind of like our endocrine system or hormones in a way too. Like in a maybe analogous way. We produce different chemicals in different situations. We tend to keep them all inside, but bacteria, you’re a little bit more giving.
DAN: Alright, so I think we’ve covered a little bit about why they’re important. But one of the things I did want to make sure that I emphasize is that the natural products field, or secondary metabolism, has been … not dominated by, but driven by drug discovery. We know that many natural product molecules are useful to humans, we really like antibiotics, we like molecules like caffeine, that do things for us that are positive. And so drug discovery has been a major driver for natural product biosynthesis. And probably a lot of the people and the scientists that we talk to will have that goal. That’s not as much our goal here at the JGI – we are not a medicinally focused organization. And so, what we do more exploration of is the use of secondary metabolism in the environment, and, sort of, understanding – like we’ve talked about – how bacteria or other organisms communicate and how and why secondary metabolites are being produced in the environment for ecological reasons.
ALISON: Can you give me an example?
DAN: Okay, so here’s an example. One of the organisms that I studied in the past were a group of bacteria called the Frankia. And so the Frankia are bacteria that live in the root nodules of plants. And these guys were sequenced, and you can see that different Frankia have different size genomes depending on what kinds of plants, and what kinds of environments, they live in. But it turns out, they have a lot of second metabolism pathways. And they use many of these pathways to communicate with other Frankia, and probably other bacteria that also live in the root nodules. And it provides – the plant provides a little house for them to live in – in the nodule, and the bacteria provide nutrients for the plant mainly by fixing nitrogen. Getting nitrogen from the environment so that the plant can grow.
Nitrogen is usually one of the, you know, important environmental [resources] that plants need to grow. And so, yeah, so these guys, it turns out, produce lots of really small molecules that they use to communicate – lots of terpenes, and little modified amino acids, and things that they secrete out into the root nodule, and help condition that nodule, and communicate with bacteria, and tell them that maybe this is a safe place to grow because we’re all here together.
ALISON: How beautiful. What a great partnership.
DAN: Yeah, I guess so. You know, it’s also another thing that we are increasingly becoming aware of in the medicinal context, is the numbers of bacteria and the things that they’re doing in the human gut. Turns out, there’s a lot of chemistry happening there – a lot of naturally identifiable secondary metabolism. And we don’t know all of the ways in which these bacteria are influencing their environment. And their environment is us! There have been stories and evidence of, you know, chemicals that make it into our bloodstream, and probably our brain, and so it’s not always clear exactly how much the bacteria are controlling us there. There are more bacteria – usually in our mouths, and certainly in our guts – than there are human beings on the planet. So there are a lot of bacteria out there. We’re definitely outnumbered. Understanding all of that chemistry is pretty important to medicine, too.
ALISON: Yeah, I mean, from the Salem witch trials, we have definitely learned a valuable historical lesson. Know your secondary metabolites!
DAN: One of the things that I did want to impress, too, is that secondary metabolism is based on primary metabolism. It’s an evolution of primary metabolism. So a lot of the genes and the DNA that’s used for secondary metabolism, they are copies and changes that nature has made. So secondary metabolism is adaptation of primary metabolism. They’re duplications of the genes, and modifications that have been made to – that nature has made to make different molecules that are related to primary metabolites. And they often use primary metabolites as building blocks to produce novel molecules. And so we’ve got glycosides being made into sugar-containing compounds, and terpenes come from isoprenes, and polyketides from the same stuff that fatty acids or lipids are made from, and amino acids build into peptides.
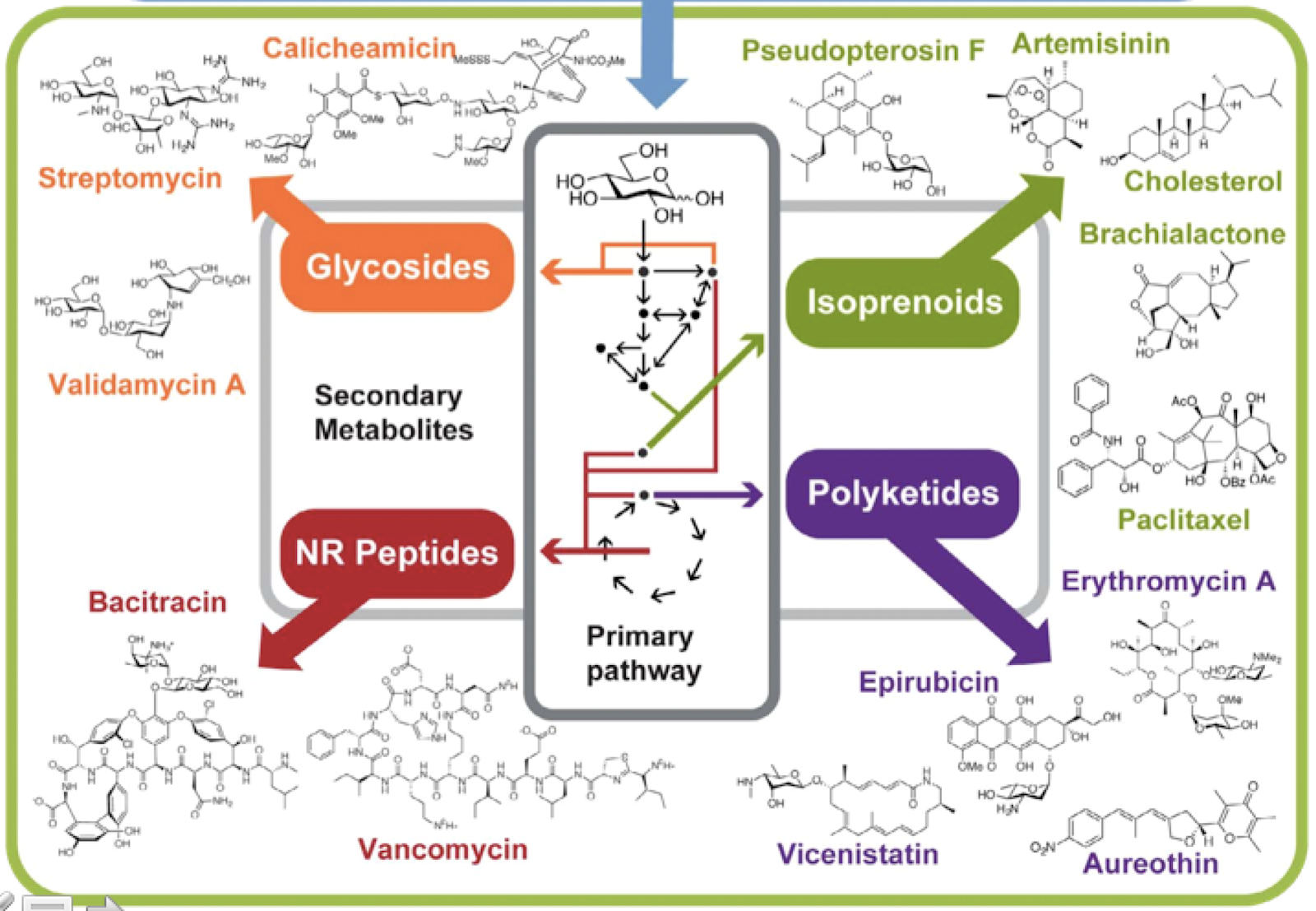
DAN: So, yeah, so historically, it’s been a little bit difficult sometimes to identify secondary metabolism, just because it is similar to primary metabolism. So if you were, you know, sequencing an organism for the first time, and you’ve never seen that organism before, you might not recognize that this weird, fatty acid synthase kind of system, like, maybe this bacteria just makes fatty acids in a weird way. Or maybe it’s a secondary metabolite. And so we’ve really only come into good science for identifying secondary metabolism as we’ve sequenced more and more and more things.
ALISON: I see. Yeah, that technology has been really crucial.
DAN: Yeah, yeah. The more data you generate, the more knowledge you can generate. So that’s why we are still sequencing lots of stuff, and why the JGI is a key driver in some of the science.
ALISON: Very cool. Yeah, and I see a lot of these secondary compounds on this slide that you have displayed. And the glycosides and peptides, a lot of them are different kinds of antibiotics, right?
DAN: Yeah, yeah, antibiotics are, like I said, a really important driver for discovery of new medicines.
ALISON: Right? I hadn’t realized that all these different kinds of secondary metabolites could also be antibiotics. And so it just feels like…
DAN: I’m not sure what the statistic is nowadays, but traditionally, somewhere in the neighborhood of 60 to 70% of all of the medicines that we use are natural products, or some simple derivatives of natural products.
ALISON: Like such a large diversity can have similar effects, I think is also really interesting. Because glycosides are shaped so differently from polyketides. At least I mean, I haven’t looked at the structures and details for a long time. But…
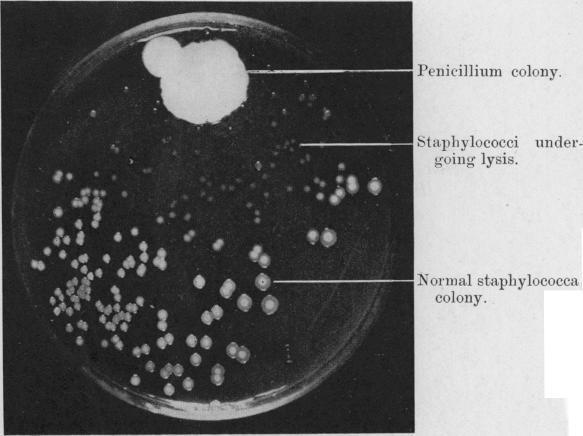
DAN: Yeah, there are a lot of ways to put together carbon molecules. Chemistry is pretty limitless. All right, so I thought we would spend a few minutes talking about a brief history of drug discovery and natural products’ role in it. All right, this is probably one of the most famous photographs in microbiology.
ALISON: Oh my gosh
DAN: Do you recognize it?
ALISON: I … do not. I feel really bad. I feel like i should.
DAN: That’s ok.
ALISON: It’s beautiful. It looks like a bunch of colonies on a petri dish. It’s a black and white photo. It almost looks like the moon, because you have all these pockmarks. But yeah, what’s going on?
DAN: All right. So this is a photograph of the plate that Alexander Fleming produced when he discovered – or was on the road to discovering – penicillin. So what we see here in this photograph is a photograph of a petri dish. And there are lots of these little white spots around, and those are Streptococcus bacteria – a kind of bacteria that you know, causes strep throat or other kinds of infections in people that are pretty unpleasant. And at the top of this photograph, or one corner of it, you see this big white blob – sort of overexposed – but this this white blob is a fungus called Penicillium. Now called Penicillium. And so this was a contaminant on his Streptococcus plate. And what do you notice about the Streptococcus spots around that?
ALISON: Oh, they’re so much smaller. Streptococcus is losing!
DAN: Yeah, so that’s because it turns out – so Fleming noticed this and it turns out that Penicillium is producing penicillin, which is inhibiting the growth of those streptococcus. And so this is one of the first discoveries of antibiotic molecules. After this, you know, Fleming went on the hunt for other similar bacteria or fungi, and found them. And that’s why penicillin saved millions of lives since then.
So, Penicillium is a fungus. But as people sort of started exploring these kinds of organisms, they noticed another somewhat similar group of organisms that I think are critically important to understanding the field of natural products. And so we’ve got another slide here, and we’re looking at a hand drawing – drawn by Selman Waxman, who was a microbiologist who was working on these things in the very early 1900s.
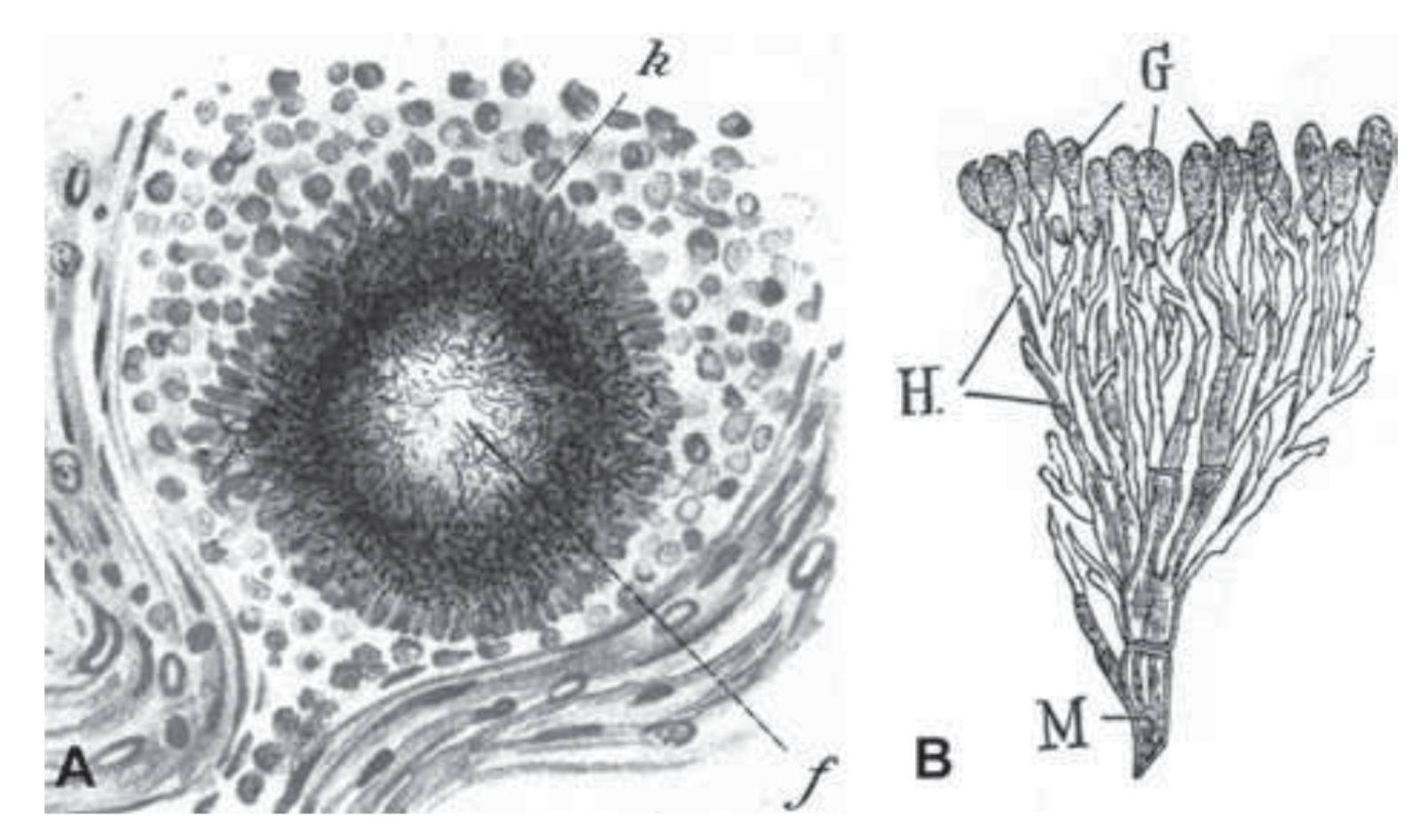
DAN: And so he has drawn what he called “actinomycetes.” So this was some … what turned out to be bacteria. He identified them as very small fungi. And this came from a growth on a cow’s cheek. Historically, before antibiotics were common, it wasn’t unusual to see sort of weird growths on livestock due to you know, bacterial or fungal infections. Yeah, okay. So he identified these things and when other organisms similar to say Penicillium were being explored, they recognized that these kinds of bacteria were producing similar molecules, antibiotic molecules, and so actinomycetes became a major source for natural products because they’re easy to find. They grow in the soil. These are the same guys that produce geosmin.
And so we are now looking at another photograph of a colony. This is a zoomed in photo of a really blown up colony of Streptomyces.
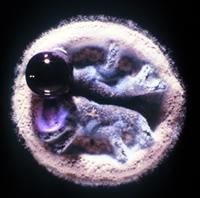
ALISON: It’s wild. It kind of looks like a sock puppet of a Yeti with a large purple lollipop attached to its mouth. We’ll have to put a picture of this on the website.
DAN: I guess looking at these is kind of like looking at pictures of clouds or “what do you see?”
ALISON: What do you see, Dan?
DAN: Well, I see a Streptomyces. So, these are bacteria that grow up in these small colonies, that are kind of leathery. And this looks fuzzy because those are all spores coming out of this thing. So these guys spread themselves with spores. And the big purple blob that you see is basically some cellular material that these guys are excreting out into their environment. These are growing on a plate, right, so it’s not their normal natural environment. They usually grow down within the soil. And that purple blob is going to be chock full of compounds that probably kill other bacteria, and give them home field advantage.
ALISON: Okay. Wow, it’s so weird how it’s all concentrated in that blob … of death.
DAN: Yeah, yeah. I mean, this is why these things were first kind of thought to be fungi. Because they’re growing in a way that’s not really, necessarily, what most people think of when you think of bacteria. You think of bacteria as sort of these single celled organisms, kind of all over. But these kinds of bacteria grow in filaments and they grow together in clumps, almost like, you know, like an organism. They have specialized cells and the ecology there is really fascinating, probably a bit under explored. But these are these are almost like, you know, like a mushroom or something that’s actually organized in the way that they grow.
ALISON: Yeah. Okay, so that bubble is probably filled with secondary metabolites.
DAN: Yep, that’s right.
ALISON: I would love to grow some stuff like that. Just to have a little collection.
DAN: All right. So, penicillin became such a critically important drug, saving many, many millions of lives. There was a drive to try to find more molecules like this. It became sort of a “golden age” of drug discovery, antibiotic discovery.
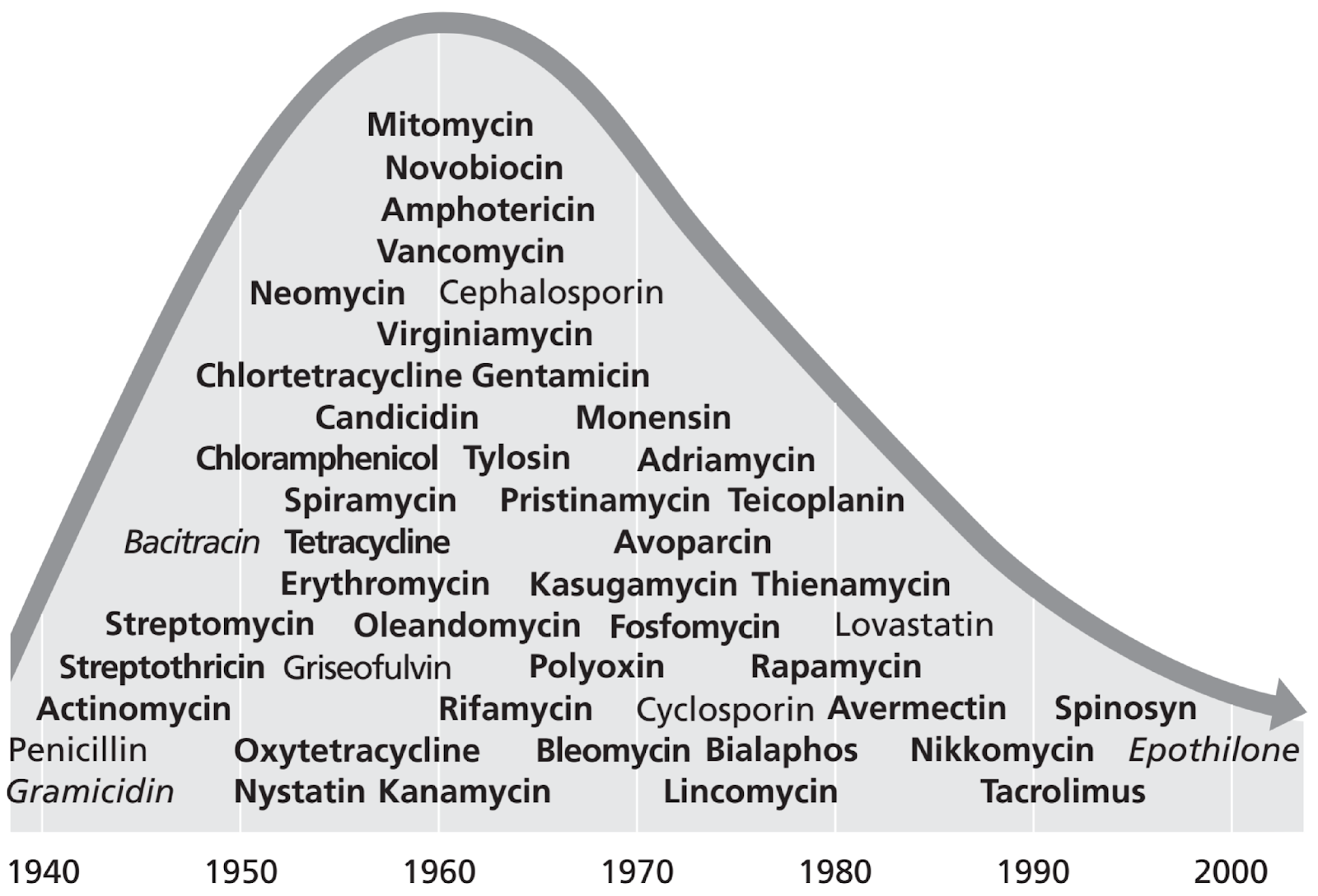
DAN: So, now we’re looking at a chart that shows different antibiotic molecules and their discoveries over a stretch from 1940 to 2000. As you can see, from the 1940s, into the 50s into the 60s, there was a huge growth – a huge explosion in new molecules discovered. This is a time when all the big drug discovery companies were creating libraries of soil bacteria they kept and –
ALISON: Yeah, vancomycin amphotericin, cephalosporin, tylosin, suka Meissen poly oxen. Some of these I definitely never heard of. Tetracycline, though I’ve heard of.
DAN: Yeah, yeah. So all of these things were discovered was what we sort of refer to as the golden age of drug discovery, where you could, you know, pick up a scoop of soil and grow some bacteria and there was a good chance you were going to find something new. That started decreasing around the 1970s, you can see on the chart, and then things get a little more difficult. And so the rate of discovery of new molecules has declined ever since then. And there are lots of reasons for that. But some of its due to just finding the same things over and over again. Once you find the low hanging fruit. You don’t you know, necessarily find – you don’t care as much about finding really similar molecules that, you know, might not be better.
ALISON: Well, so, at this point, you know, when you have all of these antibiotics being discovered, are people – are there still ailments – are there still infections that people are suffering and dying from? I think my question is: what drove people to discover more? Was it because were you already experiencing antibiotic resistance at that point?
DAN: Yeah, I’m glad you asked. One of the earliest predictions about the use of antibiotics is that eventually, antibiotic resistance is going to set in. The more you use an antibiotic, the more a bacterium is exposed to it, the greater the chance that that bacterium is going to evolve and develop some kind of resistance where it’s not affected by the antibiotic anymore. So yeah, so there was always a strong driver to find new molecules with the expectation that resistance is going to be a problem. Many of these molecules, too, hit the same target. And so there’s always a need to find new antibiotics that hit new targets because if you haven’t – So resistance comes about by usually by altering the target of the drug. So for example, a lot of antibiotics will target specific parts of a cell wall. And so if you weaken a cell wall, then the bacteria will just pop like a water balloon. But, you know, if you make modifications to your cell wall, then the antibiotic, you know, doesn’t affect it anymore. And so all of those antibiotics that were hitting the same place are also suddenly useless.
ALISON: I got it. Okay. Yeah. Smart microbes. I know they’re not actually smart. That’s anthropomorphising them.
—
DAN: I’m Dan Udwary, and you’ve been listening to Natural Prodcast, a podcast produced by the US Department of Energy Joint Genome Institute, a DOE Office of Science User Facility located at Lawrence Berkeley National Lab. You can find links to transcripts, more information on this episode, and our other episodes at naturalprodcast.com
Special thanks, as always, to my co-host, Alison Takemura. <woohoo> If you like Alison, and want to hear more science from her, check out her podcast, Genome Insider. She talks to lots of great scientists outside of secondary metabolism, and if you like what we’re doing here, you’ll probably enjoy Genome Insider too. So, check it out.
My intro and outro music are by Jahzzar.
Please help spread the word by leaving a review of Natural Prodcast on Apple podcasts, Google, Spotify, or wherever you got the podcast. If you have a question, or want to give us feedback, tweet us @JGI, or to me @danudwary. If you want to record and send us a question that we might play on air, email us at [email protected] .
And because we’re a User Facility, if you’re interested in partnering with us, we want to hear from you! We have projects in genome sequencing, DNA synthesis, transcriptomics, metabolomics, and natural products in plants, fungi, and microorganisms. If you want to collaborate, let us know!
Thanks, and see you next time!






